Of the Best Examples Which Best Describes Water Cohesion
Which example best represents the adhesion cohesion and surface tension of water. Of the following examples which best demonstrates the property of water cohesion.

Why Does Water Support Life Overview Examples Expii
Each water molecule can form four hydrogen bonds with neighbor molecules.

. This is due to water cohesion. The water drop is composed of water molecules that like to stick together an example of the property of cohesion. The strong Coulomb attraction between the molecules draws them together or makes them sticky Because the water molecules are more strongly attracted to each other than to other molecules they.
Cohesion describes waters _____. O A can of soda bursts when it is placed. Cohesion is caused by polar water molecules repelling each other.
Surface tension enables some insects to actually walk across a pond without sinking. A water drop is composed of water molecules that like to stick together-an example of the property of cohesion. A common example of cohesion is the behavior of water molecules.
Point out that a second phenomenon is also at work. In a plant there are microscopic vessels through which water runs. O Water can move up a 100-foot pine tree from the roots to the leaves.
Cohesion describes waters attraction to itself. Which best defines cohesion. It is as if the hydrogen bond is a.
Helps to transport water and nutrients from the. One example of adhesion is water climbing up a paper towel that has been dipped into a glass of water and one example of cohesion is rain falling as drops from the sky. Image of a water strider bug walking on the surface of water.
The water drop is stuck to the plant stems which is an example of the property of adhesion. Water soak also attracted to. In the picture of pine needles above the water droplets are stuck to the end of the pine needles-an example of the property of adhesion.
Examples of Cohesion. For two rain falls in droplets rather wait a. All forms of water rain in a glass etc are examples of cohesion.
Aattraction to other substances battraction to itself cability to dissolve most substances drate of cooling and heating Cohesion describes waters attraction to itself. Some examples of cohesion are. Of the following examples which best demonstrates the property of water cohesion.
When light objects float on water instead of sinking. Water molecules cling to each other and stick adhere to many surfaces. Water molecules form hydrogen bonds with cellulose in the xylem.
Water can move up a 100 foot pine tree from the roots to the leaves. Water molecules are what are called dipoles. Cohesion is caused by polar water molecules attracting each other.
Cohesion describes waters attraction to itself. Terms in this set 5 The tendency of molecules to stick together is called. Cohesion is the term for molecules of a substance sticking together.
Ice forming on the surface of water bodies in winters the ability of water to dissolve many substances water droplets forming a bead-like shape over. Biology questions and answers. The cohesive property of water is due to the hydrogen bonds which serve as a force of attraction between molecules to other molecules of water.
Water molecules cohere or stick together forming a kind of skin called surface tension. Essentially cohesion and adhesion are the stickiness that water molecules have for each other and for other substances. During adhesion water is attracted to other substances causing the positive and negative molecules of the water to be attracted to the paper.
They have an electric pole at each end of the molecule with opposite charges because the electrons in the molecule tend to congregate near the. Isotopes of an atom differ in their. One more key example of water cohesion has to do with water transport in plants.
Cohesion describes waters _____attraction to other substances attraction to itself ability to dissolve most substances rate of cooling and heating Weegy. Cohesion is caused by polar water molecules repelling and. The cohesive force governs the shape which the liquid mimics.
Cohesion with the whale for molecules of black substance sticking together One of the sheer common examples is water beading up watching a hydrophobic surface Water. O Water requires a great deal of heat to reach the point of vaporizing. Cohesion is a term used to coin the attraction between molecules of the.
O A rock skipping across the surface of a lake. Cohesive forces that occur in liquid makes intermolecular forces resist separation. Surface tension at a molecular level Water molecules want to cling onto each other.
It is responsible for waters continuous flow without breaking. Also noticeable in this picture is the effect that gravity has on the water drops. Droplets of water on a leaf such as in Figure 1.
If cohesive force is too strong the liquid can divide into small numbers of spherical beads that stand on. Cohesion usually occurs when a liquid comes in contact with a surface. Surface tension _____causes water to spread out is the ability of water to dissolve a substance creates a thin elastic layer of hydrogen atoms is caused by.
This is possible thanks to the surface tension of the water. A simple example of cohesion in action comes from the water strider below an insect that relies on surface tension to stay afloat on the surface of water. One of the most common examples is water beading up on a hydrophobic surface.
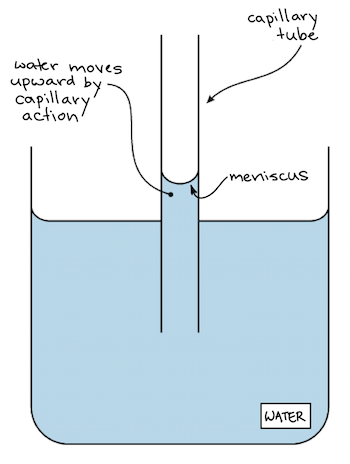
Cohesion And Adhesion Of Water Article Khan Academy

2 2e Water S Cohesive And Adhesive Properties Biology Libretexts
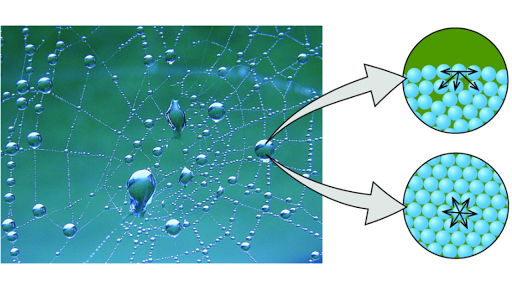
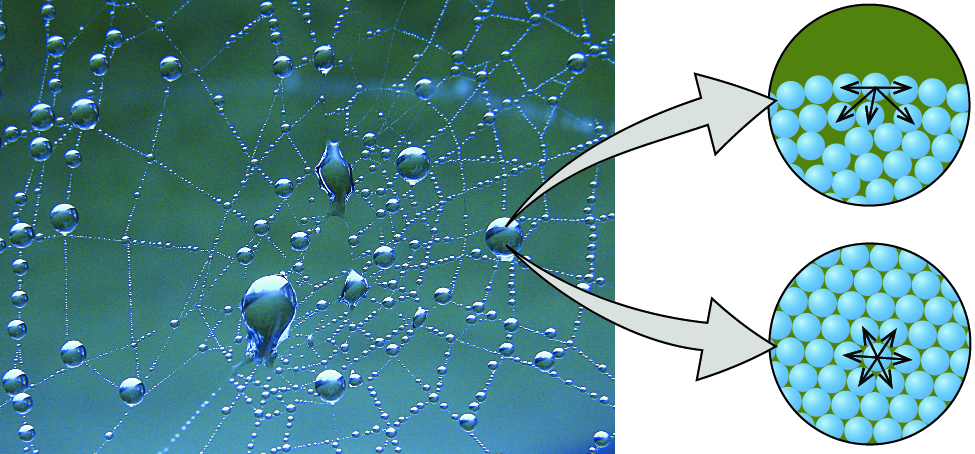
Comments
Post a Comment